In our last post we discussed diet alone cannot help to properly balance amino acids and how supplementation plays a vital role. Below is further detail:
The use of cysteine, selenium and folic acid are used to prevent depletion of the methionine-homocysteine cycle (figure 5) by L-dopa and presumably by L-tyrosine from which L-dopa is synthesized from, the immediate precursor of L-dopa. Administration of L-dopa leads to depletion of S-adenosyl-methionine (SAMe), a component of the methionine-homocysteine cycle which is the one carbon methyl donor of the body; proper levels of SAMe are needed in order for norepinephrine to be methylated to epinephrine. Long-term use of L-dopa without proper administration of amino acids of the methionine-homocysteine cycle leads to depletion of epinephrine.37
There is a, total loss of sulfur amino acid associated with treatment of Parkinsonism with L-dopa as evidenced by the loss of total glutathione which occurs.41 Glutathione is synthesized in a side chain reaction off the methioninehomocysteine cycle( see figure 5). The loss of total glutathione leads to a state where the body’s most powerful detoxifying agent (glutathione) is no longer functioning properly and is unable to neutralize further toxic insult leaving the patient in a state where more toxic damage is facilitated. All people taking L-dopa and/or L-tyrosine need to be supplemented with adequate levels of sulfur amino acids to prevent depletion of the methionine-homocysteine cycle, depletion of glutathione, depletion of epinephrine, and the other components dependent on the methionine-homocysteine cycle. While administration of any of the sulfur amino acids in the cycle is adequate if the dosing is high enough, cysteine is chosen because it is the most cost effective.
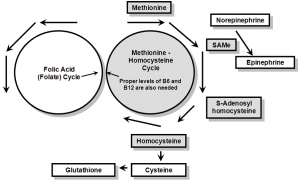 Figure 5: The methionine – homocysteine cycle , the heart of the sulfur amino acids.
Figure 5: The methionine – homocysteine cycle , the heart of the sulfur amino acids.
Selenium needs to be administered with cysteine to prevent cysteine (or any sulfur amino acids) from creating an environment that contributes to central nervous system neurotoxicity from methylmercury. Administration of cysteine can potentially facilitate concentration of methylmercury into the central nervous system.46 Selenium binds irreversibly to methylmercury in the central nervous system rendering the methylmercury biologically inactive and non-toxic.47
Folic acid is required in order to provide optimal function of the folic acid cycle which in turn prevents hyperhomocysteinemia from preventing the methioninehomocysteine cycle from functioning properly. As noted previously, without proper administration of amino acids of the methionine-homocysteine cycle leads to depletion of epinephrine. It would appear the second factor driving epinephrine levels beyond methionine-homocysteine cycle depletion is hyperhomocysteinemia. It can take 3 to 6 months for hyperhomocysteinemia to return to normal when proper levels of folate, vitamin B6 and vitamin B12 are provided for. It appears to be no coincidence that it can take 3 to 6 months for epinephrine levels to return to normal a fact that appears to parallel homocysteine improvement.
When the goal of therapy is to prevent depletion of neurotransmitters by prescription drugs or in associated situations where prescription drugs are no longer working effectively during treatment due to the neurotransmitter levels falling too low from depletion due to circumstance set up by the drug 45, the person needs to begin properly balanced amino acid therapy along with the prescription drug. While amino acid precursors when properly used alone are highly effective, a drug/amino acid combination may be desirable with severe disease, such as the suicidal patient, the catatonic patient, or the patient that is unable to take part in normal day-to-day functions such as work. Supplementing with amino acid precursors allows reuptake inhibitors to continue to function optimally without further depletion of neurotransmitter levels.
37. O-methylation and decarboxylation of alpha-methyldopa in brain and spinal cord: depletion of S-adenosylmethionine and accumulation of metabolites in catecholaminergic neurons Neuropharmacology. 1976 Jul;15(7):395-402 Lo CM, Kwok ML, Wurtman RJ.
41. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson’s disease. Exp Neurol. 2007 Feb;203(2):512-20. Epub 2006 Oct 17. Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP.
45. Role of norepinephrine in depression. Department of Psychiatry, University of Arizona, J Clin Psychiatry 2000;61 Suppl 1:5-12 Delgado PL; Moreno FA.
46. Brain, kidney and liver 203Hg-methyl mercury uptake in the rat: relationship to the neutral amino acid carrier. Pharmacol Toxicol. 1989 Jul;65(1):17-20 Aschner M.
47. Direct determination of seleno-amino acids in biological tissues by anionexchange separation and electrochemical detection. J Chromatogr A. 1995 Jul 7;706(1-2):429-36. Cavalli S, Cardellicchio N.

